Inspired by the Cu-driven conversion reaction: how anionic properties dictate the electrochemical performance of vanadium sulfide†
Abstract
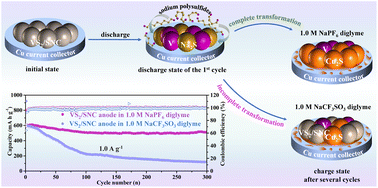
The so-called “activation process” of the VS2/SNC anode in sodium-ion batteries actually results from the Cu current collector-involved electrochemical reactions. Metallic Cu with a strong sulfiphilic property endows the VS2/SNC anode with a high reversible capacity of 586.9 mA h g−1 even after 500 cycles, but only when using the electrolyte of 1.0 M NaPF6 diglyme can such a favorable cycle stability be presented. Once the electrolyte of 1.0 M NaCF3SO3 diglyme is adopted, the device exhibits large overpotentials and its capacity decays dramatically. Examining the solvation structures and electrode/electrolyte interface properties, it is confirmed that the anionic property of the electrolyte plays a significant role in determining the electrochemical performance of metal sulfides. Triflate anions with a strong donicity not only aggravate the shuttle effect but also hinder the Na+ transport at the electrode/electrolyte interface, resulting in poor reversibility and sluggish kinetics of the anode, and such an interaction is intensified in Cu2S due to the soft acceptor characteristics of Cu (I).


 Please wait while we load your content...
Please wait while we load your content...