Biobased catalyst-free covalent adaptable networks based on CF3-activated synergistic aza-Michael exchange and transesterification†
Abstract
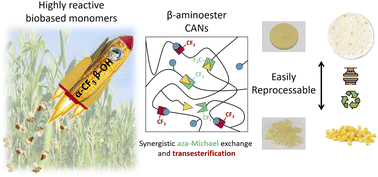
Recently, fluorine neighboring group activation emerged as a new way of promoting acid–epoxy reaction and transesterification in vitrimer materials. Pursuing this idea, the effect of a CF3 group on aza-Michael addition and aza-Michael exchange was examined and confirmed on model molecules. Following these positive results, a CAN incorporating aza-Michael bonds and CF3-activated ester functions was synthesized and compared to analogous materials deprived of any CF3 groups on the one hand, or of any hydroxyl groups on the other hand. The study of the mechanical properties of these materials highlighted the synergistic effect of the two exchange reactions and the accelerating effect of the fluorinated group. The fluorinated and hydroxylated material was shown to be reprocessable at 100 °C in 1 h under 3 tons whereas the fluorinated only and the hydroxylated only materials were respectively reprocessed at 120 °C and 150 °C. These catalyst-free CANs were synthesized from natural resources further enhancing the sustainability of these materials. This study demonstrates the potential of biobased CANs featuring fluorinated esters as low environmental impact and easily reprocessable materials.
- This article is part of the themed collections: Polymer Upcycling and 2022 Journal of Materials Chemistry A Most Popular Articles


 Please wait while we load your content...
Please wait while we load your content...