Understanding ion diffusion in anion exchange membranes; effects of morphology and mobility of pendant cationic groups†
Abstract
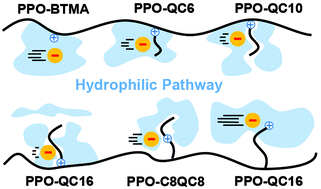
Anion exchange membranes (AEMs) are a promising low-cost alternative to cation exchange membranes (CEMs) in fuel cells. Among them, poly(2,6-dimethyl-1,4-phenylene oxide) with pendant quaternary ammonium (PPO-QA) is one of the most promising. PPO-QA membranes with flexible alkyl side-chains of varying lengths as a spacer (or extender) for QA have been studied and shown to have different performances; however, the exact underlying mechanisms have remained unclear. We study PPO-QA membranes with varying alkyl side-chains (n = 0, 6, 10, and 16 carbon atoms) with a constant QA position (near the backbone), and with the same side-chain length (n = 16) and varying QA positions on it: near the polymer backbone, in the middle, and at the end of the pendant side-chain, by molecular dynamics (MD) simulations. The calculated water diffusion coefficient in the membranes is in full agreement with experimental data. Our study on membranes with varying alkyl side-chain lengths and a constant QA position at different hydration levels shows a consistent and quantitative correlation between the anion diffusion coefficient (Da) and the hydrophilic pathway morphology. Also, at constant hydration, Da is considerably enhanced due to moving the QA position towards the end of the alkyl side-chain. However, we show that this enhancement is not due to morphological changes in the hydrophilic pathway or polymer phase. Still, the underlying mechanism is the enhanced mobility of QA, plus a lower residence time of anions close to QA. We show that the morphology of the membrane and the degree of phase separation between the hydrophobic/hydrophilic parts is almost constant, but the QA at the end of the alkyl side-chain is considerably easier to pull towards the water channels; therefore, its increased mobility and hydration result in the enhanced dynamics of the diffusing anion. Lastly, we introduce a new methodology to assess the correlation between membrane structure and ion transport rate for fuel cell membranes so that one can tune the permeation of ions (or other molecules) by two distinct mechanisms: (i) by controlling the hydrophilic pathway morphology by changing the polymer backbone structure, which determines the permeation according to the size of the diffusing moieties, and (ii) by side-chain modification, which enhances QA mobility and accordingly (selectively) increases the ion diffusion rate. We show that our methodology could successfully explain how the side-chain modification for PPO-QA membranes considerably increases the ion permeation while the methanol cross-over remains constant.


 Please wait while we load your content...
Please wait while we load your content...