A new type of sealed rechargeable lithium–lithium oxide battery based on reversible LiO2/Li2O2 interconversion†
Abstract
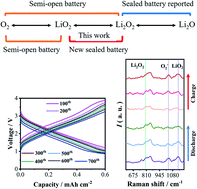
We report in this letter a new type of sealed rechargeable lithium–lithium oxide battery based on reversible interconversion between superoxide (LiO2) and lithium peroxide (Li2O2). A free-standing oxygenated group-rich reduced graphene oxide aerogel (OR-rGO) with decent mechanical properties is prepared through a hydrothermal method followed by slight drying in air at room temperature and applied as a cathode in oxygen-based beyond-intercalation Li batteries. In situ Raman spectroscopy, online electrochemical mass spectroscopy, and density functional theory calculations revealed the reversible stabilization of LiO2 by the oxygenated groups on rGO during the charge process. Based on the stability of LiO2 on oxygenated group-rich reduced graphene oxide, a new sealed lithium–lithium oxide battery via reversible Li2O2/LiO2 interconversion was realized with high operating potential up to 3.65 V and no O2 evolution. Excellent cycling stability was observed for 700 cycles at a cut-off capacitance of 0.6 mA h cm−2 and a current density of 0.6 mA cm−2.


 Please wait while we load your content...
Please wait while we load your content...