Influence of amorphous carbon interlayers on nucleation and early growth of lithium metal at the current collector-solid electrolyte interface†
Abstract
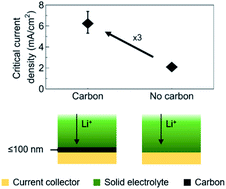
Nucleation and early growth of Li metal is critical to the performance of anode-free solid-state batteries. We report the use of amorphous carbon deposited by direct current magnetron sputtering as an intermediate layer between the Cu current collector and the Lipon solid electrolyte. The density, conductivity, and microstructure of the carbon interlayer are varied and their influence on the reversible formation and removal of the Li metal anode is investigated. It is shown that thin films of amorphous carbon act as seed layers, reducing the overpotential for Li plating and increasing the critical current density for Li plating and stripping from 2 up to 8 mA cm−2. It is further demonstrated that the ionic conductivity of the Li ions in the carbon interlayers determines their optimum thickness to be 100 nm or less, and that the initial Li loss due to interphase formation can be reduced to a few tens of nm by decreasing the density of the carbon films.


 Please wait while we load your content...
Please wait while we load your content...