Tuning electrochemical water oxidation towards ozone evolution with heterojunction anode architectures†
Abstract
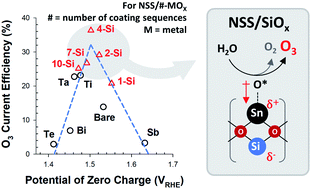
The electrochemical ozone evolution reaction (OZER) via a six-electron water–oxidation reaction can benefit the water–energy nexus. Herein, we report a straightforward heterojunction strategy to tune the OZER activity and selectivity of a Ni–Sb–SnO2 (NSS) anode, a state-of-the-art OZER electrocatalyst. The OZER overpotential (determined by scanning electrochemical microscopy) and current efficiency (CE) for NSS with metal (Ta, Ti, Si, Te, Bi, and Sb) oxide overlayers followed Sabatier's volcano relations with the potential of zero charge, which is an experimental descriptor for surface charge density. NSS/SiOx with an optimal SiOx loading occupied a position near the apex with 2.7-fold enhanced OZER CE (in 0.5 M H2SO4 at 10 mA cm−2) with retarded oxygen evolution, compared to unmodified NSS. X-ray photoelectron spectroscopy and density functional theory calculations elucidated partial charge transfer from Sn4+ to Si4+ and the overall hindrance of electron transfer to the OZER intermediates. Consequently, the reduced adsorption energies of O* and O3*, together with the strengthened O2* bonding, accounted for the improved OZER on NSS/SiOx.


 Please wait while we load your content...
Please wait while we load your content...