Interface coupling induced built-in electric fields boost electrochemical nitrate reduction to ammonia over CuO@MnO2 core–shell hierarchical nanoarrays†
Abstract
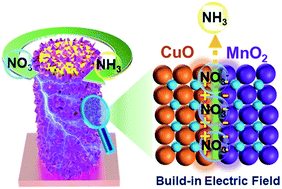
Electrochemical nitrate reduction to ammonia emerges as an attractive and promising technology for nitrate removal from water and simultaneous ammonia electrosynthesis, which demands electrocatalysts with high activity and selectivity. Herein, we designed CuO@MnO2 1D core–2D shell hierarchical nanoarrays supported on a copper foam substrate (CuO@MnO2/CF) for electrocatalytic nitrate-to-ammonia conversion. The decoration of CuO 1D nanowire arrays with MnO2 2D nanosheets brings about abundant exposed active sites and facilitates mass transfer. More importantly, the CuO/MnO2 heterogeneous nanointerface affords a well-designed built-in electric field in the interface region, which could trigger interfacial accumulation of nitrate ions and accelerate nitrate electroreduction kinetics by optimizing the chemisorption of nitrate ions or/and reaction intermediates. With these properties, an impressive electrocatalytic nitrate-to-ammonia capability was achieve over CuO@MnO2/CF (nitrate conversion: 99.38%, ammonia faradaic efficiency: 94.92%, and ammonia selectivity: 96.67%). This study opens new avenues for the rational construction of efficient electrocatalysts for nitrate removal and ammonia electrosynthesis.


 Please wait while we load your content...
Please wait while we load your content...