A nonflammable low-concentration electrolyte for lithium-ion batteries†
Abstract
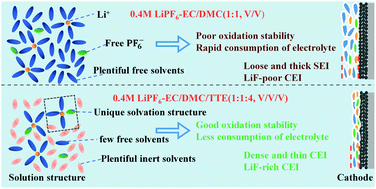
In this work, we develop a nonflammable low-concentration electrolyte (LCE) based on the Li+-solvation sheath structure. It consists of only 0.4 M LiPF6 in the mixture of ethylene carbonate (EC), dimethyl carbonate (DMC), and 1,1,2,2-tetrafluoroethyl-2,2,3,3-tetrafluoropropyl ether (TTE). Abundant incombustible TTE is used as an inert cosolvent to regulate the Li+-solvation sheath structure. With the addition of TTE, the oxidation stability of the LCE is enhanced as a result of reduced free-state solvent molecules and strengthened interactions between Li+ and EC/DMC molecules. The rate and cycling performances of Li‖LFP and Li‖NCM811 cells in 0.4 M LiPF6–EC/DMC/TTE (1 : 1 : 4, v/v/v) (0.4 M-114) are much better than in 0.4 M LiPF6–EC/DMC (1 : 1, v/v), and even better than in the conventional electrolyte (1 M LiPF6–EC/DMC (1 : 1, v/v)). The graphite‖LiNi0.5Co0.2Mn0.3O2 (NCM523) pouch cells using the 0.4 M-114 electrolyte show excellent cycling and safety performances, demonstrating the feasibility of using the nonflammable LCEs in practical applications. This work provides a nonflammable low-concentration electrolyte for lithium-ion batteries (LIBs) and provides a new insight into the development of electrolytes for LIBs.


 Please wait while we load your content...
Please wait while we load your content...