Redox catalysis-promoted fast iodine kinetics for polyiodide-free Na–I2 electrochemistry†
Abstract
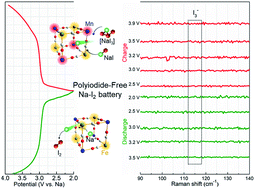
Electrochemically reversible I2/I− two-electron conversion and eliminating I3− formation are the ultimate targets for high-performance metal–iodine batteries. This work demonstrates a superior polyiodide-free Na–I2 battery. Na0.89Mn[Fe(CN)6]0.73 with open Mn and Fe co-catalytic sites anchored on a crosslinked polyaniline and polypyrrole hollow nanorod framework was used as a tandem redox catalyst to direct fast and fully reversible iodine conversion during both the battery charging and discharging processes. During kinetic discharging, the iodine species can be activated by Mn/Fe redox hotspots and the redox catalysis manipulates the reversible one-step conversion of I2 to NaI. As an intercalative host, NaxMnIII/II[FeIII/II(CN)6] facilitates the nearly full conversion of sodiated iodine to electrochemically active iodine without the leakage of soluble polyiodide intermediates during the recharging process. The significantly improved utilization of the cathode active materials and the inhibited anode parasitic reactions afford the Na–I2 cell ultrahigh cycling stability (a reversible specific capacity of 173 mA h g−1 at 500 mA g−1 after 2000 cycles with 81.4% capacity retention), high rate capability (242 and 129 mA h g−1 at 200 and 5000 mA g−1, respectively) and a distinguished flat discharge plateau of ca. 3.0 V. The redox catalysis-facilitated tunable iodine redox chemistry opens a promising avenue to unlock the full potential of metal–iodine electrochemistry.


 Please wait while we load your content...
Please wait while we load your content...