Hierarchical soot nanoparticle self-assemblies for enhanced performance as sodium-ion battery anodes†
Abstract
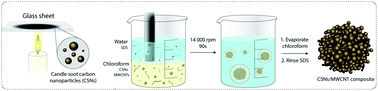
The drawbacks of amorphous hard carbon are its low conductivity and structural instability, due to its large volume change and the occurrence of side reactions with the electrolyte during cycling. Here, we propose a simple and rapid method to address these disadvantages; we used an emulsion solvent–evaporation method to create hierarchically structured microparticles of hard carbon nanoparticles, derived from soot, and multi-walled-carbon-nanotubes at a very low threshold of 2.8 wt%. These shrub-ball like microparticles have well-defined void spaces between different nanostructures of carbon, leading to an increased surface area, lower charge-resistance and side reactions, and higher electronic conductivity for Na+ insertion and de-insertion. They can be slurry cast to assemble Na+ anodes, exhibiting an initial discharge capacity of 713.3 mA h g−1 and showing long-term stability with 120.8 mA h g−1 at 500 mA g−1 after 500 cycles, thus outperforming neat hard carbon nanoparticles by an order of magnitude. Our work shows that hierarchical self-assembly is attractive for increasing the performance of microparticles used for battery production.


 Please wait while we load your content...
Please wait while we load your content...