Ca-ion modified vanadium oxide nanoribbons with enhanced Zn-ion storage capability†
Abstract
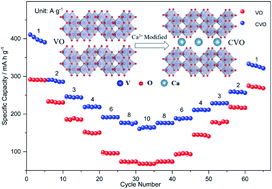
Vanadates with high theoretical capacity and various crystal structures are deemed promising layered cathode materials for aqueous Zn ion batteries (ZIBs). Yet, their capacity and rate properties are still unsatisfactory as a result of their slow electron and ion transport rate. In this work, we report a one-pot hydrothermal method to introduce the divalent Ca-ion into V3O7 (CVO) nanoribbons, which markedly boosts their Zn-ion storage capacity and rate capability. The introduction of Ca2+ can remarkably enhance the conductivity and expand the interlayer spacing of V3O7 (VO), enabling a swift diffusion of Zn2+ and transfer of electrons. Hence, the Zn//CVO battery based on the CVO cathode obtained a satisfactory capacity of 432.3 mA h g−1 at a current density of 1 A g−1, excellent rate performance (165.5 mA h g−1 at 10 A g−1), and good cycle life (78.5% capacity retention after 800 cycles). Furthermore, the fabricated Zn//CVO battery delivered a high energy density of 340.0 W h kg−1 and a peak power density of 8.7 kW kg−1. This Ca2+ modification strategy may provide a new approach to the design and synthesis of other layered transition metal oxides for energy storage.


 Please wait while we load your content...
Please wait while we load your content...