Efficient production of hydrogen from H2S via electrolysis using a CoFeS2 catalyst†
Abstract
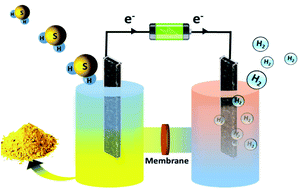
Transformation of noxious and undesirable industrial waste H2S into hydrogen (H2) via electrolysis will be a game-changing strategy. Herein, we have developed a cost-effective and highly stable nanorod embedded wheat grain CoFeS2 in conjugation with a nitrogen containing carbon framework for pure H2 production from H2S by reduction at the cathode and the sulfide oxidation reaction (SOR) at the anode. The proposed catalyst exhibits the lowest onset potential of 0.23 V vs. RHE to drive H2S electrolysis which is 1.0 V lower than the thermodynamic potential of electrochemical water splitting (1.23 V). Moreover, it demonstrates 97.8% H2 faradaic efficiency with remarkable durability up to 120 h. These outcomes demonstrate a promising future prospective of H2S as a cost-effective H2 source towards a more sustainable economy simultaneously eliminating the environmental pollutant.


 Please wait while we load your content...
Please wait while we load your content...