Intercalation of cobalt cations into Co9S8 interlayers for highly efficient and stable electrocatalytic hydrogen evolution†
Abstract
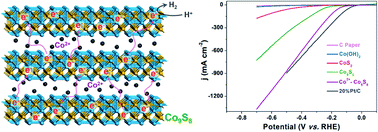
Non-noble metal based electrocatalysts for the hydrogen evolution reaction (HER) hold great potential for commercial applications. However, effective design strategies are greatly needed to manipulate the catalyst structures to achieve high activity and stability comparable to those of noble-metal based electrocatalysts. Herein, we present a facile route to synthesize layered Co9S8 intercalated with Co cations (Co2+-Co9S8) (with interlayer distance up to 1.08 nm) via a one-step solvothermal method. Benefiting from a large interlayer distance and efficient electron transfer between layers, the Co2+-Co9S8 hybrid shows outstanding electrocatalytic hydrogen evolution performance in an acid electrolyte. The electrocatalytic performance is even better than that of 20% Pt/C at the <−0.54 V region with an overpotential of 86 mV at a current density of 10 mA cm−2 in 0.5 mol L−1 H2SO4. More importantly, the system can maintain excellent stability for more than 12 h without obvious decay. This study not only presents a novel and efficient approach to synthesize cobalt sulfide intercalated with Co cations for stable electrocatalytic HER but also provides an avenue for the design of intercalated materials used in other energy applications.


 Please wait while we load your content...
Please wait while we load your content...