CO oxidation on MgAl2O4 supported Irn: activation of lattice oxygen in the subnanometer regime and emergence of nuclearity-activity volcano†
Abstract
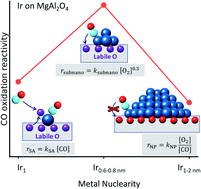
CO oxidation on Pt group metals is affected by the metal size and reducibility of the oxide support. Here, we report that Ir supported on MgAl2O4, traditionally considered non-reducible, exhibits properties similar to reducible oxides when the Ir size is in the subnanometer regime. To show this effect, we synthesized subnanometer Ir clusters and compared their properties to single atoms and nanoparticles (1–1.5 nm). The CO oxidation activity is highest on Ir0.6–0.8nm while showing distinctly different reaction orders in CO and O2 (0, +0.4), than single atoms (1, 0) and nanoparticles (−1, +1). Microcalorimetry, in situ X-ray absorption, and infrared spectroscopies show that the CO-saturated Ir0.6–0.8nm clusters could adsorb and activate O2 despite binding CO more strongly than nanoparticles. Density functional theory calculations on CO saturated Ir4 clusters suggest that the increased activity is due to the ability to activate O2 on oxygen vacancies at the Ir–MgAl2O4 interface. The findings show the important effect of the metal nuclearity on the support and catalyst properties and can guide future design of CO oxidation catalysts.


 Please wait while we load your content...
Please wait while we load your content...