Single-phase proton- and electron-conducting Ag-organic coordination polymers for efficient CO2 electroreduction†
Abstract
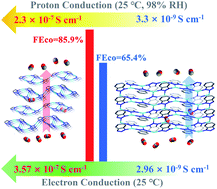
Here a single-phase proton- and electron-conducting metal–organic coordination polymer (MOCP) electrocatalyst for the CO2 reduction reaction (CO2RR) was constructed by using Ag as the metal center and 1H-1,2,3-triazole (Tz) as the ligand. To evaluate the effects of tuning the conductivity of Ag-MOCP on CO2RR performances, another Ag-MOCP was selected for comparison by using 1H-benzotriazole (Btz) as the ligand. The proton conductivity for Ag-MOCP-Tz is 2.3 × 10−7 S cm−1, 70-fold that for Ag-MOCP-Btz with 3.3 × 10−9 S cm−1 at 25 °C and 98% RH. And the electron conductivity of Ag-MOCP-Tz (3.57 × 10−7 S cm−1) is 120 times that of Ag-MOCP-Btz (2.96 × 10−9 S cm−1) at 25 °C. Meanwhile the FECO of Ag-MOCP-Tz is 85.9% at −0.98 V vs. RHE, which is better than that of Ag-MOCP-Btz with 65.4%.


 Please wait while we load your content...
Please wait while we load your content...