New insights into the mechanism of cation migration induced by cation–anion dynamic coupling in superionic conductors†
Abstract
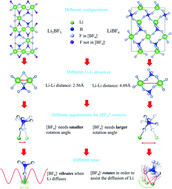
Understanding the ion diffusion mechanism is one of the key preconditions for designing superionic conductors in solid state lithium batteries and many other energy devices. Besides the single-cation vacancy/interstitial-assisted and multi-cation concerted migration, the dynamic coupling between cations and anions has been detected in systems containing SO42−, PS43−, BH4−, etc., and is also believed to relate to the cation migration. Here, a theory of the cation–anion interactions is presented, which classifies the coupling into vibrational-type and rotational-type according to the motion behavior of anion groups and their contributions to the ionic conductivity are analyzed. By screening the dynamic characteristics of common anion groups, a new system containing anion-assisted Li migration is identified in LiBF4 and Li2BF5. Based on a thorough analysis of ab initio molecular dynamics simulations, the Li+ migration induced by BF4− motion is separated from the hopping steps, and the vibration- and the rotation-assisted-migration are identified as the two characteristic processes to realize the long-range diffusion of Li+ ions.


 Please wait while we load your content...
Please wait while we load your content...