A review of sodium chloride-based electrolytes and materials for electrochemical energy technology
Abstract
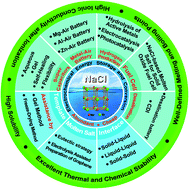
Sodium chloride (NaCl), as one of the most naturally abundant compounds, plays an irreplaceable role in industrial development and human life. In recent years, NaCl has received significant attention in the field of electrochemical energy due to its unique physicochemical properties, environmental friendliness and low cost. This paper reviews the recent progress in the utilization of NaCl in electrochemical energy technologies, such as supercapacitors, batteries, fuel cells, metal–air batteries, hydrogen production, and electrochemical desalination. The advances in NaCl applications in electrolytes mainly include the preliminary studies on the thermodynamic properties of NaCl solutions, aqueous NaCl-based electrolytes and NaCl-based gel electrolytes for supercapacitors, metal–air batteries, water splitting to produce hydrogen, capacitive desalination (CDI) and desalination battery, and NaCl-based composite electrolytes for solid oxide fuel cells. The regulation principles and methods of NaCl in the microstructure of electrode materials, mainly involving the NaCl-based template method with the assistance of freezing and gel technologies, NaCl-based molten salt method guided by the by electrolysis and eutectic point theory, and NaCl-based interface regulation technology, are discussed through a comprehensive analysis of the literature. Several key technical challenges are also summarized and analyzed with proposing several possible future research directions for overcoming the challenges.


 Please wait while we load your content...
Please wait while we load your content...