Elucidating the reaction pathway of crystalline multi-metal borides for highly efficient oxygen-evolving electrocatalysts†
Abstract
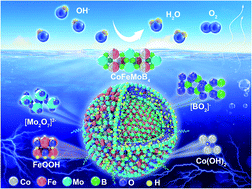
Understanding the fundamental principle of catalytic performance and the mechanism of multimetal-based electrocatalysts is essential for the rational design of advanced renewable energy systems. Here, highly crystalline MMMoB4 (M = Fe, Co) compounds with controllable compositions of multiple active metal atoms and polyacene-type boron networks were synthesized delicately by a one-step high-pressure technique to explore electrocatalytic selectivity and activity. CoFeMoB4 and Co2MoB4 are revealed to be highly active and durable oxygen evolution reaction (OER) electrocatalysts under alkaline conditions. The mutually promotive activation of metals with amorphous clusters and ultra-small grains on the surface are responsible for the enhanced activity of CoFeMoB4. More specifically, Co and Fe coupling in CoFeMoB4 facilitates surface reconstruction into active Co hydroxide and Fe oxyhydroxide, in contrast to Co oxyhydroxide in Co2MoB4 and Fe oxides in Fe2MoB4. Dissolving Mo may provide potential space for adsorbing hydroxyl, and the optimized electronic structure with boron is mainly responsible for the long-term durability. In contrast, Mo atoms are responsible for hydrogen evolution reaction (HER) properties, and the optimized d-band center and density of states at the Fermi level make Co2MoB4 a superior HER catalyst. Our findings provide insight into distinguishing the catalytic pathway of multi-metal borides with improved OER activity and different roles of Mo and Co/Fe in the HER and OER.


 Please wait while we load your content...
Please wait while we load your content...