Elucidation of the structural and optical properties of metal cation (Na+, K+, and Bi3+) incorporated Cs2AgInCl6 double perovskite nanocrystals†‡
Abstract
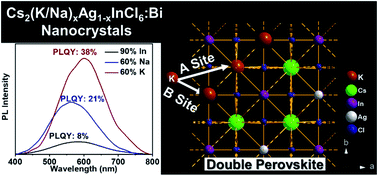
This study presents series of direct band gap Pb-free double perovskite Cs2AgInxBi1−xCl6, Cs2NaxAg1−xInCl6:Bi and Cs2KxAg1−xInCl6:Bi nanocrystal systems [Cs2B′(I)B′′(III)Cl6] synthesised using a colloidal hot-injection route. The structural properties investigated using powder XRD, TEM, solid state NMR and materials modelling approaches demonstrate that the incorporation of K+ cations into the double perovskite nanocrystal structure occurs simultaneously on both the Cs (A) site and Ag (B′(I)) positions within a series of closely related cubic and monoclinic structures. As a result of defect passivation, significant improvements in the photoluminescence quantum yield (PLQY) of ∼4.7× and ∼1.8× are exhibited in comparison to the Cs2AgInxBi1−xCl6, and Cs2NaxAg1−xInCl6:Bi nanocrystal systems, respectively. Materials modelling using the Ab Initio Random Structure Search (AIRSS) method, and the GIPAW DFT calculation of the NMR parameters from the derived structural realisations, shows that K+ incorporation induces significant short-range structural disorder and multi-phase formation. This is highlighted by the large 133Cs and 39K chemical shift dispersion characterising the MAS NMR data. Density of States (DoS) calculations describing these AIRSS generated structures suggest that increasing ionic character and reduced structural rigidity are strongly correlated with A site substitution of the K+ cation into these cubic and monoclinic phases. The 39K MAS NMR data reveals that the increasing PLQY performance maps directly with the K+ incorporation into the cubic CsKyAg1−yInCl6 phase supporting B site occupancy which is observed to be maximized at a 60 ml% K+ incorporation level. However, additional evidence indicates that low level K+ substitution primarily targets A site occupancy in a surface passivation role. The improvement to the optical properties induced by K+ and Na+ incorporation is rationalised in terms of increased covalent character and structural rigidity associated with decreased Cs+, Na+ and K+ cation mobility, as evidenced by the large (∼2 orders of magnitude) variation in the 133Cs T1 data across each compositional range.


 Please wait while we load your content...
Please wait while we load your content...