Persistence length of α-helical poly-l-lysine†
Abstract
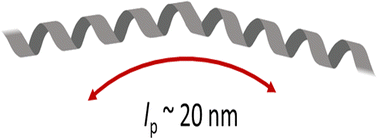
The α-helix has a significant role in protein function and structure because of its rigidity. In this study, we investigate the persistence length, lp, of α-helical poly-L-lysine, PLL, for two molecular weights. PLL experiences a random coil–helix transition as the pH is raised from 7 to 12. Using light scattering experiments to determine the radius of gyration (Rg), hydrodynamic radius, (Rh), the shape factor (Rg/Rh), and second virial coefficient (A2), and circular dichroism to determine the helical content, we find the structure and lp of PLL as a function of pH (7.4–11.4) and ionic strength (100–166 mM). With increasing pH, we find an increase in lp from 2 nm to 15–21 nm because of α-helix formation. We performed dissipative particle dynamics (DPD) simulations and found a similar increase in lp. While this lp is less than that predicted by molecular dynamics simulations, it is consistent with other experimental results, which quantify the mechanics of α-helices. By determining the mechanics of helical polypeptides like PLL, we can further understand their implications to protein function.


 Please wait while we load your content...
Please wait while we load your content...