N-Alkylimidazolium carboxylates as a new type of catanionic surface active ionic liquid: synthesis, thermotropic behavior and micellization in water†
Abstract
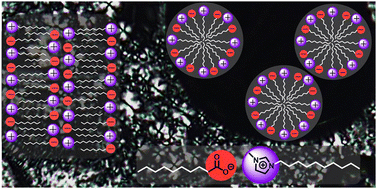
Aiming at a new type of salt-free CASAIL (Catanionic Surface Active IL) for electrochemical applications or emulsifiers, dispersants, and foaming or antifoaming agents, we combined mesogenic anions (carboxylate) and cations (imidazolium) of similar shape and size to synthesize a series of congruent ion pairs of 1-alkyl-3-methylimidazolium alkylcarboxylates [Cnmim][Cm−1COO] (n = 10–16, m = 10–16). With particular focus on alkyl chain length varieties in both, imidazolium cations and carboxylate anions (n/m), the self-assembly in the bulk phase and in solution was investigated by differential scanning calorimetry (DSC), polarized optical microscopy (POM), X-ray diffraction (XRD) experiments and surface tension measurements. Our results revealed that the presence of long alkyl chains on both the cation n and anion m leads to improved thermal stability of the bulk material while maintaining broad lamellar (SmA) mesophases. In water, we observed a strong and linear decrease of log(cmc) for increasing both the carboxylate anion and imidazolium cation chain length due to the increasing hydrophobic effect. Surprisingly, for both thermotropic behavior and micellization, the chain length of the carboxylate anion had a stronger impact than the chain length of the imidazolium cation, indicating its greater surface activity and tendency to form micelles.


 Please wait while we load your content...
Please wait while we load your content...