Reversible stability of colloids switched by CO2 based on polyhexamethylene guanidine†
Abstract
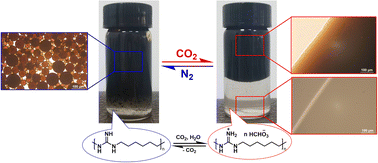
The stability of a colloid, including emulsion and polymer latex, can be destroyed irreversibly by the addition of salt. Using the CO2 stimulus, amines can be converted into organic ammonium salts reversibly, which can access the switching of colloids. Polyhexamethylene guanidine (PHMG), was chosen as a switchable amine. The conductivity of PHMG aqueous solution switched by adding and removing CO2. Surface tension measurements verified that, under CO2, the critical micelle concentration of sodium dodecyl benzene sulfonate (SDBS) decreased from 1.0 × 10−3 to 5.0 × 10−4 M with the addition of PHMG. The crude oil emulsion containing SDBS and PHMG was destroyed and restored reversibly by the treatment with CO2 and N2. The polystyrene latex also occurred an obvious stratification after sparging with CO2 and returned a homogeneous phase upon bubbling N2. This study is intended to pave the way for colloids which has reversible stability in response to CO2 stimulation.


 Please wait while we load your content...
Please wait while we load your content...