On-demand gelation of ionic liquids using photoresponsive organometallic gelators†
Abstract
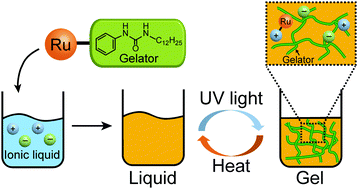
The reversible formation of ionic liquid gels, or ionogels, upon external stimuli could improve their versatility and expand their application scope in electronic, biomedical, and micro-engineering systems. Herein, we developed organometallic compounds that release low-molecular-weight gelators upon photoirradiation, which facilitate the on-demand photogelation of ionic liquids (ILs). The chemical formulae of the gelator-coordinated complexes are [Ru(C5H5)L]X (L = C6H5NHCONHC12H25; X = PF6, B(CN)4). Each of the complexes were ILs that are easy to synthesize and miscible in ILs. By adding a small amount of the complex, various ILs were transformed to gels upon UV photoirradiation. The PF6 salt allowed the photogelation of ILs with coordinating substituents, whereas the B(CN)4 salt allowed the photogelation of non-coordinating ILs, albeit the reaction was slower. These gels underwent the reverse reaction and liquefied back when heated, and the photogelation was repeatable for ILs with coordinating cations.


 Please wait while we load your content...
Please wait while we load your content...