Investigating the entropic nature of membrane-mediated interactions driving the aggregation of peripheral proteins
Abstract
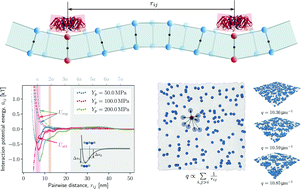
Peripheral membrane-associated proteins are known to accumulate on the surface of biomembranes as a result of membrane-mediated interactions. For a pair of rotationally-symmetric curvature-inducing proteins, membrane mechanics at the low-temperature limit predicts pure repulsion. On the other hand, temperature-dependent entropic forces arise between pairs of stiff-binding proteins suppressing membrane fluctuations. These Casimir-like interactions have thus been suggested as candidates for attractive forces leading to aggregation. With dense assemblies of peripheral proteins on the membrane, both these abstractions encounter short-range and multi-body complications. Here, we make use of a particle-based membrane model augmented with flexible peripheral proteins to quantify purely membrane-mediated interactions and investigate their underlying nature. We introduce a continuous reaction coordinate corresponding to the progression of protein aggregation. We obtain free energy and entropy landscapes for different surface concentrations along this reaction coordinate. In parallel, we investigate time-dependent estimates of membrane entropy corresponding to membrane undulations and coarse-grained director field and how they change dynamically with protein aggregation. Congruent outcomes of the two approaches point to the conclusion that for low surface concentrations, interactions with an entropic nature may drive the aggregation. But at high concentrations, enthalpic contributions due to concerted membrane deformation by protein clusters are dominant.


 Please wait while we load your content...
Please wait while we load your content...