Brownmillerite-type Ca2Fe0.75Co1.25O5 as a robust electrocatalyst for the oxygen evolution reaction under neutral conditions†
Abstract
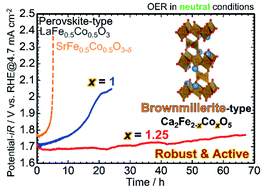
Towards the development of active and robust electrocatalysts for the oxygen evolution reaction (OER) to realize water splitting and CO2 electroreduction by renewable energies under practical conditions, brownmillerite-type composite oxide Ca2Fe2−xCoxO5 was synthesized by a sol–gel method and examined for the OER under neutral conditions. A pure brownmillerite-type Ca2Fe2−xCoxO5 phase, which was categorized as an oxygen-deficiency-ordered perovskite-type structure, was synthesized in a range of x = 0–1.25. Brownmillerite-type Ca2FeCoO5 showed higher activity and durability for the OER in a neutral solution than perovskite-type LaFe0.5Co0.5O3 and SrFe0.5Co0.5O3−δ. Furthermore, the durability of Ca2Fe2−xCoxO5 was drastically improved by increasing the Co content from x = 1 to 1.25. Ca2Fe0.75Co1.25O5 was remarkably durable for about 70 h during the OER in a neutral solution whereas Ca2FeCoO5 lost activity within 20 h, and perovskite-type oxides lost activity within a few hours. The detailed analysis of structures and compositions of local regions of Ca2Fe2−xCoxO5 and their changes by the reaction based on electron microscopy clarified the important role of the brownmillerite structure itself in catalytic activity under neutral conditions, while, under alkaline conditions, it has been known that the amorphous phase derived from brownmillerite mainly played the role of active species.


 Please wait while we load your content...
Please wait while we load your content...