A two-dimensional perovskite oxyfluoride Pb3Fe2O5F2 as a catalyst for electrochemical oxidation of water to oxygen†
Abstract
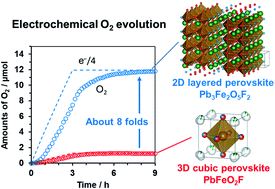
A two-dimensional (2D) perovskite oxyfluoride Pb3Fe2O5F2, deposited on a conductive glass substrate, exhibited activity for electrochemical oxidation of water. This 2D oxyfluoride electrode could oxidize water forming O2 at a relatively low potential of +1.7 V vs. RHE with a faradaic efficiency of almost unity; the O2 evolution activity was nearly 8-fold higher compared to that for a 3D bulk PbFeO2F analogue prepared in a similar manner.


 Please wait while we load your content...
Please wait while we load your content...