Microwave-induced controlled-isomerization during glucose conversion into lactic acid over a Sn-beta catalyst†
Abstract
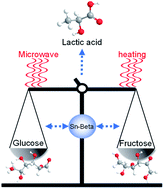
Microwave was introduced for the controlled-isomerization of glucose, facilitating its efficient conversion into lactic acid with a very high yield of 68.3 wt% at 180 °C for 30 min over a “monolithic” Sn-beta catalyst in water. 1H/13C NMR and H/D exchange experiments revealed the percentages of furanose (27.1%) and pyranose (72.1%), which reached completely different tautomeric equilibria in the microwave field compared with those with conventional heating under otherwise similar reaction conditions. The dominance of pyranose among the tautomers favored the reverse-isomerization, which resulted in the highly efficient production of lactic acid by restricting the side reactions under microwave irradiation. Thus, altered fructose tautomeric equilibria along with the controlled reverse-isomerization, which were realized by microwave irradiation due to its special non-thermal effect, facilitated lactic acid production from bio-feedstocks.


 Please wait while we load your content...
Please wait while we load your content...