Esterification of 1-hexene and acetic acid catalyzed by a modified resin with a Lewis acid†
Abstract
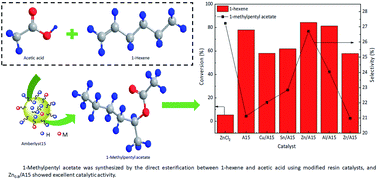
The activity and selectivity of products for the esterification of C5+ linear olefins catalyzed by a resin catalyst are the bottlenecks that must be overcome. In this work, a series of modified resin catalysts which have both Brønsted acid sites and Lewis acid sites were prepared by using SnCl2, CuCl2, ZnCl2, AlCl3 and ZrCl4 as modifying reagents, and they were used to catalyze the direct esterification of 1-hexene to prepare 1-methylpentyl acetate. The morphology, structure, composition, and thermal stability of the catalysts were characterized by FT-IR, SEM, SEM-EDS, XRF, XPS, Py-IR, BET and TG techniques. Among the different modified resin catalysts, Zn/A15 possessed excellent catalytic activity. The results showed that the catalyst activity of the modified resin was related to the type of Lewis acid, which determined the balance relationship between Lewis acid strength and Brønsted acid strength and the affinity of metal ions with acetic acid. Further investigation confirmed that the Brønsted acid site was the major active center in the esterification of 1-hexene. Under optimum reaction conditions, the conversion of 1-hexene could reach 90.24%, and the selectivity of 1-methylpentyl acetate was 31.35%. In addition, a reuse experiment indicated that introducing Zn2+ on the surface of A15 could increase the stability of C–S, and the main reason for the decrease of catalytic activity was the loss of –SO3H and Zn2+.


 Please wait while we load your content...
Please wait while we load your content...