anti-Selective synthesis of β-boryl-α-amino acid derivatives by Cu-catalysed borylamination of α,β-unsaturated esters†
Abstract
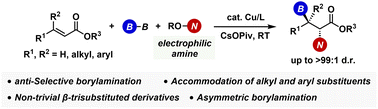
A copper-catalysed regio- and diastereoselective borylamination of α,β-unsaturated esters with B2pin2 and hydroxylamines has been developed to deliver acyclic β-boryl-α-amino acid derivatives with high anti-diastereoselectivity (up to >99 : 1), which is difficult to obtain by the established methods. A chiral phosphoramidite ligand also successfully induces the enantioselectivity, giving the optically active β-borylated α-amino acids. The products can be stereospecifically transformed into β-functionalised α-amino acids, which are of potent interest in medicinal chemistry.


 Please wait while we load your content...
Please wait while we load your content...