Angularly fused diaza-dinaphthopyrenes: regio-selective synthesis, crystal structures and isomer-dependent mechanochromic fluorescent properties†
Abstract
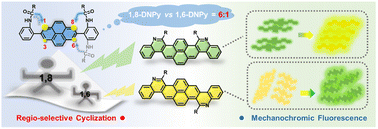
We report a one-pot synthesis of a series of unprecedented angular-fused diaza-dinaphthopyrene isomers (1,8-DNPy and 1,6-DNPy) in high yields, which are enabled by regio-selective Bischler–Napieralski cyclization to fuse two quinolone rings either on the same or opposite faces of a pyrene core. Benefiting from the high reactivity of the 1- and 8-positions of the pyrene ring, steric effect from substitution and remarkably different dipole moments, high ring closure selectivity for the 1,8-form vs. the 1,6-form up to 6 : 1 is achieved with ease of separation. With differentiated molecular symmetry, conformation, intermolecular interactions and aromaticity, the two kinds of regio-isomers exhibit distinct single-crystal structures and optoelectronic properties. Impressively, isomer-dependent mechanochromic fluorescent properties of these 2D-azaacenes are identified, which are unique in their turn-on fluorescence feature and contrasting spectral shifts. These findings allow facile and modular access to regio-specific 2D-N-heteroarenes, which provide a way to create innovative optical sensors with improved sensitivity and fruitful fluorescent properties.
- This article is part of the themed collection: Most popular 2022 materials and energy articles


 Please wait while we load your content...
Please wait while we load your content...