Dual reactivity disulfide bridging reagents; enabling new approaches to antibody fragment bioconjugation†
Abstract
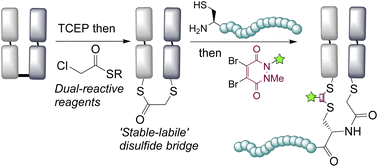
Disulfide bridging, also known as disulfide stapling, is a powerful strategy for the construction of site-selective protein bioconjugates. Here we describe the first examples of a new class of such reagents, containing a ‘stable-labile’ design. These dual-reactive reagents are designed to form a stable bond to one cysteine and a labile bond to the second; resulting in a robust attachment to the protein with one end of the bridge, whilst the other end serves as a reactive handle for subsequent bioconjugation. By incorporating thioesters into these bridges, we demonstrate that they are primed for native chemical ligation (NCL) with N-terminal cysteines; offering an alternative to the requirement for C-terminal thioesters for use in such ligations. Alternatively, the use of hydrazine as the ligating nucleophile enables a separate cargo to be attached to each cysteine residue, which are exploited to insert variably cleavable linkers. These methodologies are demonstrated on an antibody fragment, and serve to expand the scope of disulfide bridging strategies whilst offering a convenient route to the construction of multifunctional antibody fragment conjugates.
- This article is part of the themed collection: 2022 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...