Substrate-controlled C–H or C–C alkynylation of cyclopropanes: generation of aryl radical cations by direct light activation of hypervalent iodine reagents†
Abstract
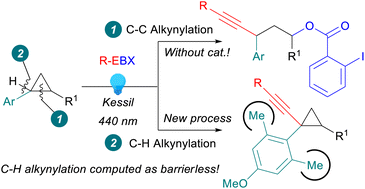
We report the first oxidative C–H alkynylation of arylcyclopropanes. Irradiation of ethynylbenziodoxolone (EBX) reagents with visible light at 440 nm promoted the reaction. By the choice of the aryl group on the cyclopropane, it was possible to completely switch the outcome of the reaction from the alkynylation of the C–H bond to the oxyalkynylation of the C–C bond, which proceeded without the need for a catalyst, in contrast to previous works. The oxyalkynylation could also be extended to aminocyclopropanes as well as styrenes. Computations indicated that the C–H activation became a favoured nearly barrierless process in the presence of two ortho methyl groups on the benzene ring.


 Please wait while we load your content...
Please wait while we load your content...