A manganese(i)tricarbonyl-catalyst for near room temperature alkene and alkyne hydroarylation†
Abstract
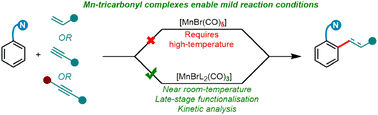
Developing more efficient catalytic processes using abundant and low toxicity transition metals is key to enable their mainstream use in synthetic chemistry. We have rationally designed a new Mn(I)-catalyst for hydroarylation reactions that displays much improved catalytic activity over the commonly used MnBr(CO)5. Our catalyst, MnBr(CO)3(MeCN)2, avoids the formation of the off-cycle manganacycle-(CO)4 species responsible for low catalyst activity, allowing near room temperature hydroarylation of alkenes and alkynes with broad functional group tolerance including late stage functionalisation and diversification of bioactive molecules.


 Please wait while we load your content...
Please wait while we load your content...