InI3-catalyzed polyene cyclization of allenes and its application in the total synthesis of seven abietane-type diterpenoids†
Abstract
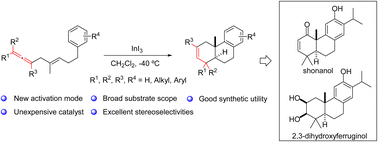
A novel polyene cyclization using the allene group as the initiator has been successfully developed. This methodology provides an efficient strategy for the construction of an abietane-type tricyclic skeleton with a functionalizable C2–C3 double bond and features a wide substrate scope and excellent stereoselectivities. Potential utility of this approach has been well demonstrated by the collective total synthesis of seven abietane-type diterpenoids. Specifically, (±)-2,3-dihydroxyferruginol and (±)-2,3-dihydroxy-15,16-dinor-ent-pimar-8,11,13-triene were synthesized for the first time.


 Please wait while we load your content...
Please wait while we load your content...