A photoinduced transient activating strategy for late-stage chemoselective C(sp3)–H trifluoromethylation of azines†
Abstract
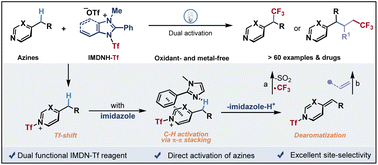
The direct functionalization of C(sp3)–H bonds is an ultimately ideal synthetic strategy with high atom economy and step efficiency. However, the direct trifluoromethylation of electron-deficient heteroaryl adjacent C(sp3)–H bonds remains a formidable challenge. We have described a transient activating strategy involving a Tf-shift process and π–π stacking interaction for catalyst-free direct benzylic C(sp3)–H trifluoromethylation of azines, such as pyridine, pyrimidine, quinoline, dihydropyridinone, tetrahydroisoquinoline and tetrahydroquinazoline, with an air-stable crystalline imidazolium sulfonate reagent IMDN-Tf. This bench-stable cationic reagent offers a scalable and practical protocol for the late-stage modification of drug molecules with high site selectivity, which avoids the prefunctionalization and the use of stoichiometric metals and strong oxidants. Furthermore, comprehensive mechanistic studies revealed the determining effect of π–π stacking for the activation of azinylic C(sp3)–H bonds.


 Please wait while we load your content...
Please wait while we load your content...