Isomer recognition by dynamic guest-adaptive ligand rotation in a metal–organic framework with local flexibility†
Abstract
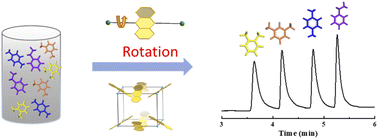
Local flexibility in a metal–organic framework is intriguing for reconstructing a microenvironment to distinguish different guest molecules by emphasizing their differences. Herein, guest-adaptive flexibility is observed in a metal–organic framework for efficiently discriminating aromatic isomers. Microcrystal electron diffraction directly reveals that the anthracene rings can rotate around the single bond with the adsorption of guest molecules. Disorder transformation of the ligand enables the preferential adsorption of ethylbenzene over other xylene isomers. Especially, a coated capillary column combining single/multi-component adsorption confirms a unique separation order of ethylbenzene > p-xylene > m-xylene > o-xylene with excellent selectivities, which has not been reported in other materials. Density functional theory calculations and the calculated Hirshfeld surface of guest molecules in the framework demonstrate that a guest-induced splint-like confinement structure makes the main contribution to such separation performance. This finding will provide a rational strategy for molecular recognition utilizing the local flexibility of metal–organic frameworks.


 Please wait while we load your content...
Please wait while we load your content...