Ligand-enabled oxidation of gold(i) complexes with o-quinones†
Abstract
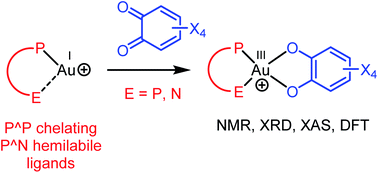
Chelating P^P and hemilabile P^N ligands were found to trigger the oxidation of Au(I) complexes by o-benzoquinones. The ensuing Au(III) catecholate complexes have been characterized by NMR spectroscopy, single crystal X-ray diffraction and X-ray absorption spectroscopy. They adopt tetracoordinate square-planar structures. Reactivity studies substantiate the reversibility of the transformation. In particular, the addition of competing ligands such as chloride and alkenes gives back Au(I) complexes with concomitant release of the o-quinone. DFT calculations provide insight about the structure and relative stability of the Au(I) o-quinone and Au(III) catecholate forms, and shed light on the 2-electron transfer from gold to the o-quinone.


 Please wait while we load your content...
Please wait while we load your content...