Unified synthesis of multiply arylated alkanes by catalytic deoxygenative transformation of diarylketones†
Abstract
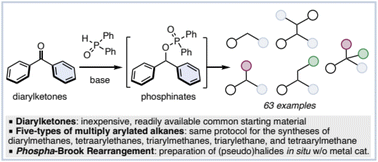
A deoxygenative transformation of diarylketones leading to multiply arylated alkanes was developed. Diarylketones were reacted with diphenylphosphine oxide resulting in a phospha-Brook rearrangement, followed by palladium-catalyzed cross-couplings or a Friedel–Crafts type alkylation to afford the corresponding multiply arylated alkanes. A variety of diarylketones can be converted to multiply arylated alkanes such as diarylmethanes, tetraarylethanes, and triarylmethanes by reduction, dimerization, and arylation in one pot. Furthermore, a one-pot conversion from arylcarboxylic acids to diarylmethanes and tetraarylethanes, and a synthesis of tetraarylmethane and triphenylethane using sequential coupling reactions are also presented.


 Please wait while we load your content...
Please wait while we load your content...