A cooperative adsorbent for the switch-like capture of carbon dioxide from crude natural gas†
Abstract
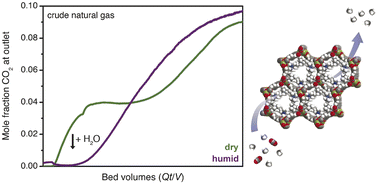
Natural gas constitutes a growing share of global primary energy due to its abundant supply and lower CO2 emission intensity compared to coal. For many natural gas reserves, CO2 contamination must be removed at the wellhead to meet pipeline specifications. Here, we demonstrate the potential of the diamine-appended metal–organic framework ee-2–Mg2(dobpdc) (ee-2 = N,N-diethylethylenediamine; dobpdc4− = 4,4′-dioxidobiphenyl-3,3′-dicarboxylate) as a next-generation CO2 capture material for high-pressure natural gas purification. Owing to a cooperative adsorption mechanism involving formation of ammonium carbamate chains, ee-2–Mg2(dobpdc) can be readily regenerated with a minimal change in temperature or pressure and maintains its CO2 capacity in the presence of water. Moreover, breakthrough experiments reveal that water enhances the CO2 capture performance of ee-2–Mg2(dobpdc) by eliminating “slip” of CO2 before full breakthrough. Spectroscopic characterization and multicomponent adsorption isobars suggest that the enhanced performance under humid conditions arises from preferential stabilization of the CO2-inserted phase in the presence of water. The favorable performance of ee-2–Mg2(dobpdc) is further demonstrated through comparison with a benchmark material for this separation, zeolite 13X, as well as extended pressure cycling. Overall, these results support continued development of ee-2–Mg2(dobpdc) as a promising adsorbent for natural gas purification.
- This article is part of the themed collection: 2022 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...