Photomediated reductive coupling of nitroarenes with aldehydes for amide synthesis†
Abstract
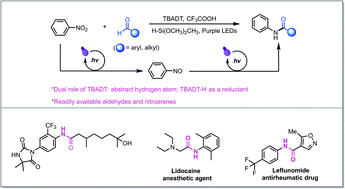
In view of the widespread significance of amide functional groups in organic synthesis and pharmaceutical studies, an efficient and practical synthetic protocol that avoids the use of stoichiometric activating reagents or metallic reductants is highly desirable. A straight-forward pathway to access amides from abundant chemical feedstock would offer a strategic advantage in the synthesis of complex amides. We herein disclose a direct reductive amidation reaction using readily available aldehydes and nitroarenes enabled by photo-mediated hydrogen atom transfer catalysis. It avoids the use of metallic reductants and production of toxic chemical waste. While aldehydes represent a classic class of electrophilic synthons, the corresponding nucleophilic acyl radicals could be directly accessed by photo hydrogen atom transfer catalysis, enabling polarity inversion. Our method provides an orthogonal strategy to conventional amide couplings, tolerating nucleophilic substituents such as free alcohols and sensitive functional groups to amines such as carbonyl or formyl groups. The synthetic utilization of this reductive amidation is demonstrated by the late-stage modification of complex biologically active molecules and direct access of drug molecules leflunomide and lidocaine.


 Please wait while we load your content...
Please wait while we load your content...