Photochemistry of pyruvic acid is governed by photo-induced intermolecular electron transfer through hydrogen bonds†
Abstract
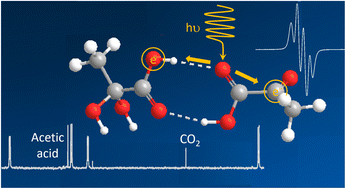
Despite more than 85 years of research, the mechanism behind the photodecarboxylation of pyruvic acid remains elusive. Most studies focused on the gas and liquid phase of diluted solutions of pyruvic acid to understand the impact of sun light on the degradation of this molecule in the atmosphere. By analyzing concentrated supercooled solutions at 77 K, we demonstrate that instead of decarboxylating, the pyruvic acid molecule plays the role of electron donor and transfers an electron to an acceptor molecule that subsequently degrades to form CO2. We show that this electron transfer occurs via hydrogen bonding and that in aqueous solutions of pyruvic acid, the hydrated form is the electron acceptor. These findings demonstrate that photo-induced electron transfer via hydrogen bonding can occur between two simple carboxylic acids and that this mechanism governs the photochemistry of pyruvic acid, providing unexplored alternative pathways for the decarboxylation of photo-inactive molecules.


 Please wait while we load your content...
Please wait while we load your content...