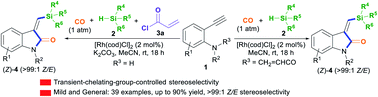
The transient-chelating-group-controlled stereoselective Rh(i)-catalyzed silylative aminocarbonylation of 2-alkynylanilines: access to (Z)-3-(silylmethylene)indolin-2-ones†
Abstract
A new method involving mild acryl transient-chelating-group-controlled stereoselective Rh(I)-catalyzed silylative aminocarbonylation of 2-alkynylanilines with CO and silanes is presented for producing (Z)-3-(silylmethylene)indolin-2-ones. Upon using an acryl transient chelating group, 2-alkynylanilines undergo an unprecedented alkyne cis-silylrhodation followed by aminocarbonylation to assemble (Z)-3-(silylmethylene)indolin-2-ones. Mechanistic studies show that acryl transient chelating effects result in the key alkyne cis-silylrhodation process.


 Please wait while we load your content...
Please wait while we load your content...