Radical generation enabled by photoinduced N–O bond fragmentation†
Abstract
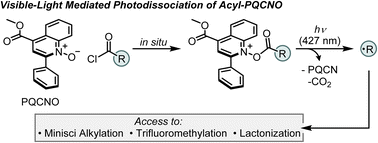
Recent advances in synthetic chemistry have seen a resurgence in the development of methods for visible light-mediated radical generation. Herein, we report the development of a photoactive ester based on a quinoline N-oxide core structure, that provides a strong oxidant in its excited state. The heteroaromatic N-oxide provides access to primary, secondary, and tertiary radical intermediates, and its application toward the development of a photochemical Minisci alkylation is reported.


 Please wait while we load your content...
Please wait while we load your content...