Carbocation catalysis in confined space: activation of trityl chloride inside the hexameric resorcinarene capsule†
Abstract
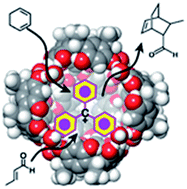
Carbocation catalysis can be performed inside the confined space of the hexameric resorcinarene capsule. The inner cavity of the capsule can host the trityl carbocation, which catalyses the Diels–Alder reaction between dienes and unsaturated aldehydes. Experimental results and in silico calculations show that the hexameric resorcinarene capsule C6 can promote the formation of the trityl carbocation from trityl chloride through the cleavage of the carbon–halogen bond promoted by OH⋯X− hydrogen bonding. Here it is shown that the combination of the nanoconfined space and the latent carbocation catalysis provides a convenient complementary strategy for the typical carbocation catalysis. The latent strategy bypasses the typical pitfalls associated with active carbocations and provides control of the reaction efficiency in terms of reaction rate, conversion, and selectivity.
- This article is part of the themed collection: Most popular 2022 catalysis articles


 Please wait while we load your content...
Please wait while we load your content...