Construction of a quaternary stereogenic center by asymmetric hydroformylation: a straightforward method to prepare chiral α-quaternary amino acids†
Abstract
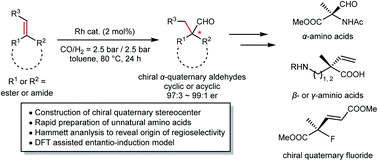
The construction of chiral quaternary carbon stereocenters has been a long-standing challenge in organic chemistry. Particularly, α-quaternary amino acids that are of high importance in biochemistry still lack a straightforward synthetic method. We here reported a hydroformylation approach to access chiral quaternary stereogenic centers, which has been a long-standing challenge in transition metal catalysis. α,β-Unsaturated carboxylic acid derivatives undergo hydroformylation with a rhodium catalyst to generate an α-quaternary stereocenter under mild conditions. By using this method, a variety of chiral α-quaternary amino acids could be synthesized with satisfactory enantioselectivity. In-depth investigation revealed that the regioselectivity is dramatically influenced by the electronic properties of the substituents attached to the target C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond. By applying NMR and DFT analyses, the chiral environment of a rhodium/Yanphos complex was depicted, based on which a substrate-catalyst interaction model was proposed.
C bond. By applying NMR and DFT analyses, the chiral environment of a rhodium/Yanphos complex was depicted, based on which a substrate-catalyst interaction model was proposed.


 Please wait while we load your content...
Please wait while we load your content...