Enantiomeric β-sheet peptides from Aβ form homochiral pleated β-sheets rather than heterochiral rippled β-sheets†
Abstract
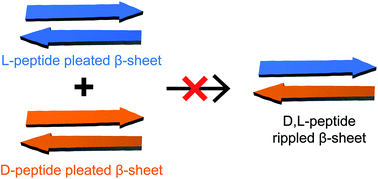
In 1953, Pauling and Corey postulated “rippled” β-sheets, composed of a mixture of D- and L-peptide strands, as a hypothetical alternative to the now well-established structures of “pleated” β-sheets, which they proposed as a component of all-L-proteins. Growing interest in rippled β-sheets over the past decade has led to the development of mixtures of D- and L-peptides for biomedical applications, and a theory has emerged that mixtures of enantiomeric β-sheet peptides prefer to co-assemble in a heterochiral fashion to form rippled β-sheets. Intrigued by conflicting reports that enantiomeric β-sheet peptides prefer to self-assemble in a homochiral fashion to form pleated β-sheets, we set out address this controversy using two β-sheet peptides derived from Aβ17–23 and Aβ30–36, peptides 1a and 1b. Each of these peptides self-assembles to form tetramers comprising sandwiches of β-sheet dimers in aqueous solution. Through solution-phase NMR spectroscopy, we characterize the different species formed when peptides 1a and 1b are mixed with their respective D-enantiomers, peptides ent-1a and ent-1b. 1H NMR, DOSY, and 1H,15N-HSQC experiments reveal that mixing peptides 1a and ent-1a results in the predominant formation of homochiral tetramers, with a smaller fraction of a new heterochiral tetramer, and mixing peptides 1b and ent-1b does not result in any detectable heterochiral assembly. 15N-edited NOESY reveals that the heterochiral tetramer formed by peptides 1a and ent-1a is composed of two homochiral dimers. Collectively, these NMR studies of Aβ-derived peptides provide compelling evidence that enantiomeric β-sheet peptides prefer to self-assemble in a homochiral fashion in aqueous solution.
- This article is part of the themed collection: 2022 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...