Magnesium-stabilised transition metal formyl complexes: structures, bonding, and ethenediolate formation†
Abstract
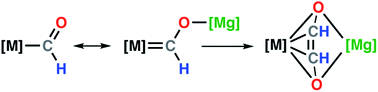
Herein we report the first comprehensive series of crystallographically characterised transition metal formyl complexes. In these complexes, the formyl ligand is trapped as part of a chelating structure between a transition metal (Cr, Mn, Fe, Co, Rh, W, and Ir) and a magnesium (Mg) cation. Calculations suggest that this bonding mode results in significant oxycarbene-character of the formyl ligand. Further reaction of a heterometallic Cr–Mg formyl complex results in a rare example of C–C coupling and formation of an ethenediolate complex. DFT calculations support a key role for the formyl-intermediate in ethenediolate formation. These results show that well-defined transition metal formyl complexes are potential intermediates in the homologation of carbon monoxide.


 Please wait while we load your content...
Please wait while we load your content...