Electronic structure analysis of electrochemical CO2 reduction by iron-porphyrins reveals basic requirements for design of catalysts bearing non-innocent ligands†
Abstract
Electrocatalytic CO2 reduction is a possible solution to the increasing CO2 concentration in the earth’s atmosphere, because it enables storage of energy while using the harmful CO2 feedstock as a starting material. Notably, iron(II) tetraphenylporphyrin, [FeII(TPP)]0 (TPP2− = tetraphenylporphyrin tetra-anion diradical), and its derivatives have been established as one of the most promising families of homogeneous catalysts for CO2 reduction into CO. Our earlier work has demonstrated that [Fe(TPP)]2−, a catalytically active species, is best described as an Fe(II) center antiferromagnetically coupled with a TPP4− diradical. In fact, [Fe(TPP)]2− represents a prototypical example of a diverse array of highly efficient molecular catalysts that feature non-innocent ligands. To obtain valuable insights for future catalyst design, their outstanding catalytic performance warrants an investigation aimed at elucidating the role played by the ligand non-innocence in the reaction. To this end, the reactivity of [Fe(TPP)]2− was first investigated in detail by using density functional theory calculations, and the theoretical results were then validated by reproducing available experimental kinetic and thermodynamic data. Further in-depth analyses pinpointed the electronic-structure feature of the non-innocent TPP ligand that is responsible for the high efficiency of the reaction. Finally, we analyzed the electronic-structure evolution found for the reactions catalyzed by ten related representative non-innocent systems. Our results revealed that for the reactions under consideration, the reducing equivalents are stored on the non-innocent ligand, while CO2 functionalization takes place at the metal center. Therefore, all of the transformations invariably entail two synchronized electron-transfer events: (1) a metal-to-CO2 transfer and (2) a ligand-to-metal electron transfer. The former is affected by σ-donation from the metal dz2 orbital to the CO2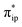 orbital, and the latter is facilitated by orbital coupling between the ligand and the metal center. Our results suggested that ligand non-innocence plays a fundamental role in stabilizing highly active intermediates while realizing high product selectivity for CO2 reduction and that the metal–ligand cooperativity is essential to the high reaction kinetics. On the basis of these findings, we proposed fundamental requirements for design of catalysts with non-innocent ligands.
orbital, and the latter is facilitated by orbital coupling between the ligand and the metal center. Our results suggested that ligand non-innocence plays a fundamental role in stabilizing highly active intermediates while realizing high product selectivity for CO2 reduction and that the metal–ligand cooperativity is essential to the high reaction kinetics. On the basis of these findings, we proposed fundamental requirements for design of catalysts with non-innocent ligands.



 Please wait while we load your content...
Please wait while we load your content...