Electrochemical synthesis of N,N′-disubstituted indazolin-3-ones via an intramolecular anodic dehydrogenative N–N coupling reaction†
Abstract
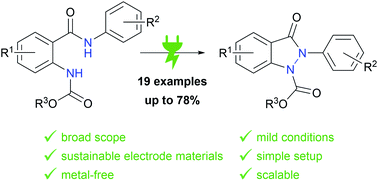
The use of electricity as a traceless oxidant enables a sustainable and novel approach to N,N′-disubstituted indazolin-3-ones by an intramolecular anodic dehydrogenative N–N coupling reaction. This method is characterized by mild reaction conditions, an easy experimental setup, excellent scalability, and a high atom economy. It was used to synthesize various indazolin-3-one derivatives in yields up to 78%, applying inexpensive and sustainable electrode materials and a low supporting electrolyte concentration. Mechanistic studies, based on cyclic voltammetry experiments, revealed a biradical pathway. Furthermore, the access to single 2-aryl substituted indazolin-3-ones by cleavage of the protecting group could be demonstrated.


 Please wait while we load your content...
Please wait while we load your content...