π-Extension of heterocycles via a Pd-catalyzed heterocyclic aryne annulation: π-extended donors for TADF emitters†
Abstract
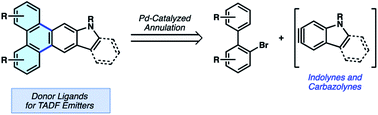
We report the annulation of heterocyclic building blocks to access π-extended polycyclic aromatic hydrocarbons (PAHs). The method involves the trapping of short-lived hetarynes with catalytically-generated biaryl palladium intermediates and allows for the concise appendage of three or more fused aromatic rings about a central heterocyclic building block. Our studies focus on annulating the indole and carbazole heterocycles through the use of indolyne and carbazolyne chemistry, respectively, the latter of which required the synthesis of a new carbazolyne precursor. Notably, these represent rare examples of transition metal-catalyzed reactions of N-containing hetarynes. We demonstrate the utility of our methodology in the synthesis of heterocyclic π-extended PAHs, which were then applied as ligands in two-coordinate metal complexes. As a result of these studies, we identified a new thermally-activated delayed fluorescence (TADF) emitter that displays up to 81% photoluminescence efficiency, along with insight into structure–property relationships. These studies underscore the utility of heterocyclic strained intermediates in the synthesis and study of organic materials.


 Please wait while we load your content...
Please wait while we load your content...